Correlation between corrosion behavior and grain boundary characteristics by lab-based DCT
6061 Al alloy (Al-Si-Mg-Cu) is widely used for structural components in aerospace, transportation, and marine engineering because of its ease of fabrication and relatively high strength. Copper is added to improve the strength, however, this simultaneously reduces the resistance to intergranular corrosion. Therefore, a thorough understanding of crystallographic effects on both intergranular and pitting corrosion is important to optimize corrosion resistance by controlling the microstructure.
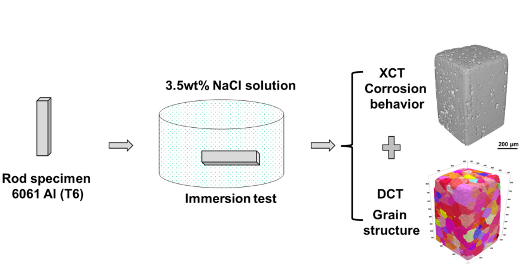
A group of researchers, headed by Prof. Nik Chawla from Purdue University, investigated the corrosion behavior of a peak aged 6061 Al alloy through a combination of lab-based DCT to determine the grain structure and absorption contrast tomography to follow the corrosion when the alloy was immersed in a 3.5 wt% NaCl solution. They found that pitting corrosion happens at the position of both Al5FeSi inclusions and Mg2Si precipitates, while the intergranular corrosion is actually caused by the galvanic cell between grain boundary precipitates and the matrix.
Not all grain boundaries are equally susceptible to intergranular corrosion
The 3D distribution of grain boundary characteristics was quantitatively analyzed by DCT and correlated with the intergranular corrosion behavior. Investigations revealed that grain boundaries with misorientation angles smaller than 25◦ or larger than 45◦ exhibited stronger resistance to intergranular corrosion, while grain boundaries with misorientation angles between 25◦ and 45◦ corroded more easily.
Materials Characterization
By the same authors
3D investigation of grain boundaries in industrial grade aluminum
